Table of Contents
Introduction
Semiconductors are the unsung heroes powering nearly every modern device, from smartphones to cars and even satellites. But what exactly are they? Semiconductors sit at the heart of technology, acting as the backbone for innovations that shape our daily lives. Let’s dive into the fascinating world of semiconductors and discover their secrets.
Check out our guide on How Transistors Work: A Complete Guide with Examples and Applications
What is a Semiconductor?
A semiconductor is a material that has a conductivity level between that of an insulator (like rubber) and a conductor (like copper). These unique properties make semiconductors incredibly versatile in electronics.
They work by controlling the flow of electrical current, which is essential for powering devices like computers, TVs, and LEDs.
Now, let’s further understand this with an example of a stadium entrance and people. Let’s imagine the stadium entrance as a conductor and the people as current. Now we can see a large group of people passing through the gates and into the stadium easily, in the same manner in which conductors allow electricity to pass through them easily.
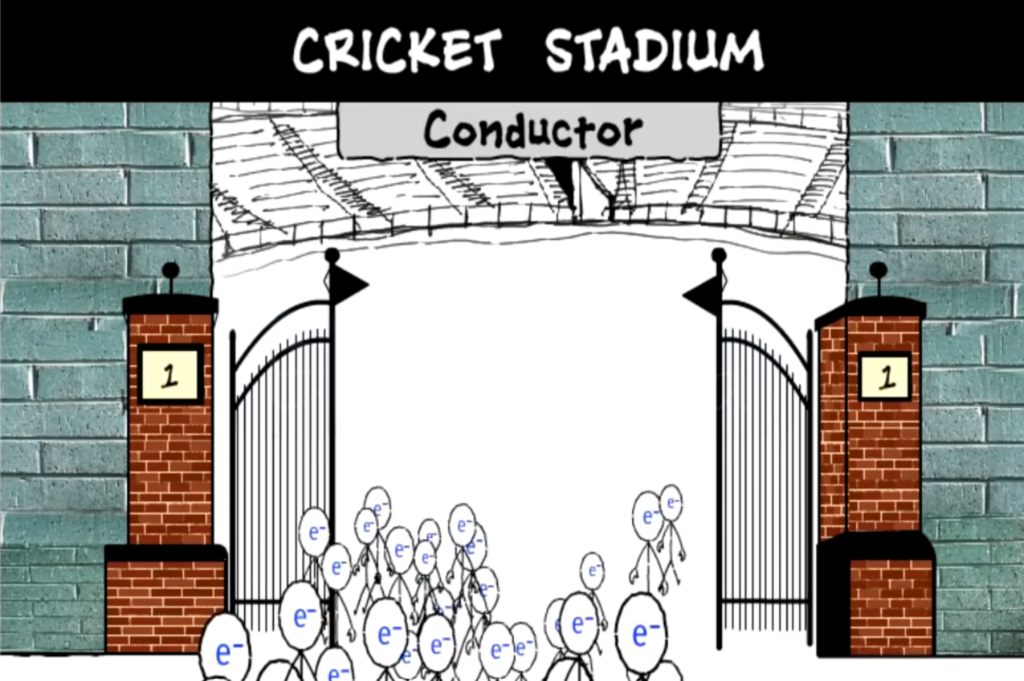
However, here we see the crowd of people moving past the entrance one by one, slowly in a queue. Now, why is that? That’s because the current in a semiconductor passes through it partially, but continuously.
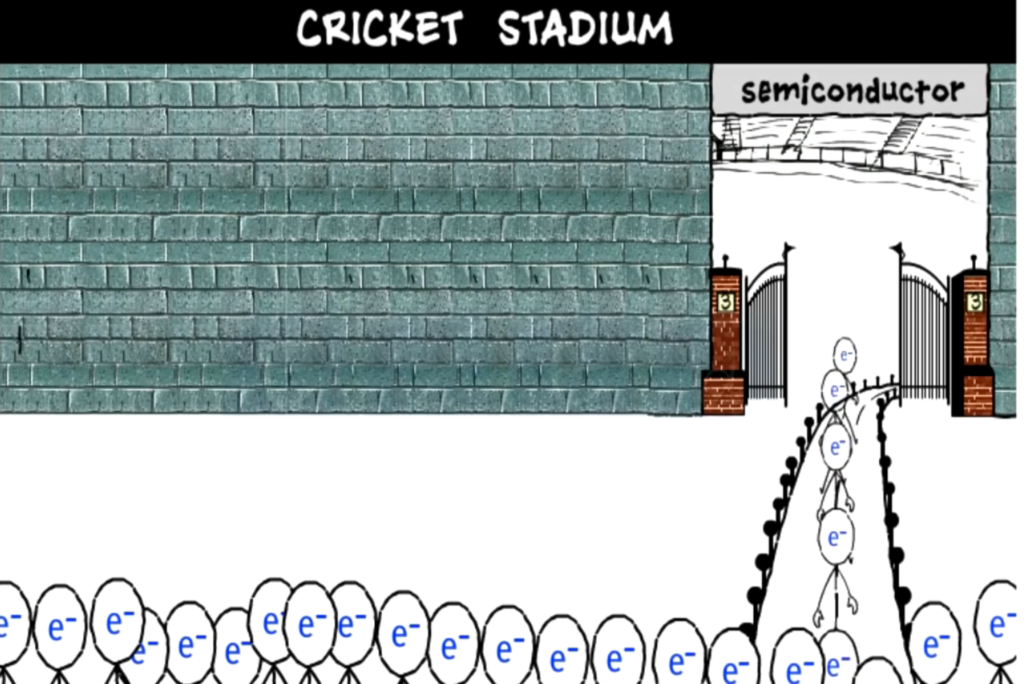
In the case of insulators that are bad conductors of electricity, we can see the stadium entrance, which we have assumed to be an insulator, is blocked, thus not allowing the crowd of people which we have assumed to be as current to pass through it.
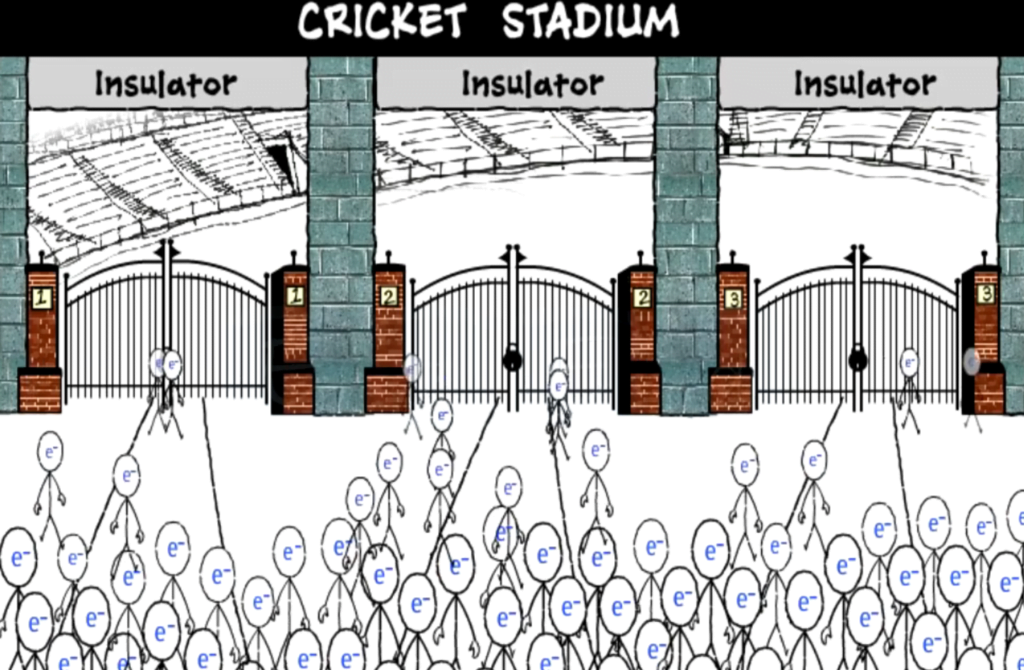
The term semiconducting was used for the first time by Alexander Wolter in 1782, however, the first documented observation of a semiconductor effect is that of Michael Faraday in 1833, who noticed that the resistance of silver sulphide decreased with temperature, which was different then the temperature dependent’s observed in metals.
Types Of Semiconductor
Semiconductors are further divided into intrinsic and extrinsic semiconductors. Depending on the structure, properties, etc.
Intrinsic semiconductors
- These are pure semiconductors, like silicon or germanium, with no added impurities.
- Their conductivity is naturally low but can increase with heat or light.
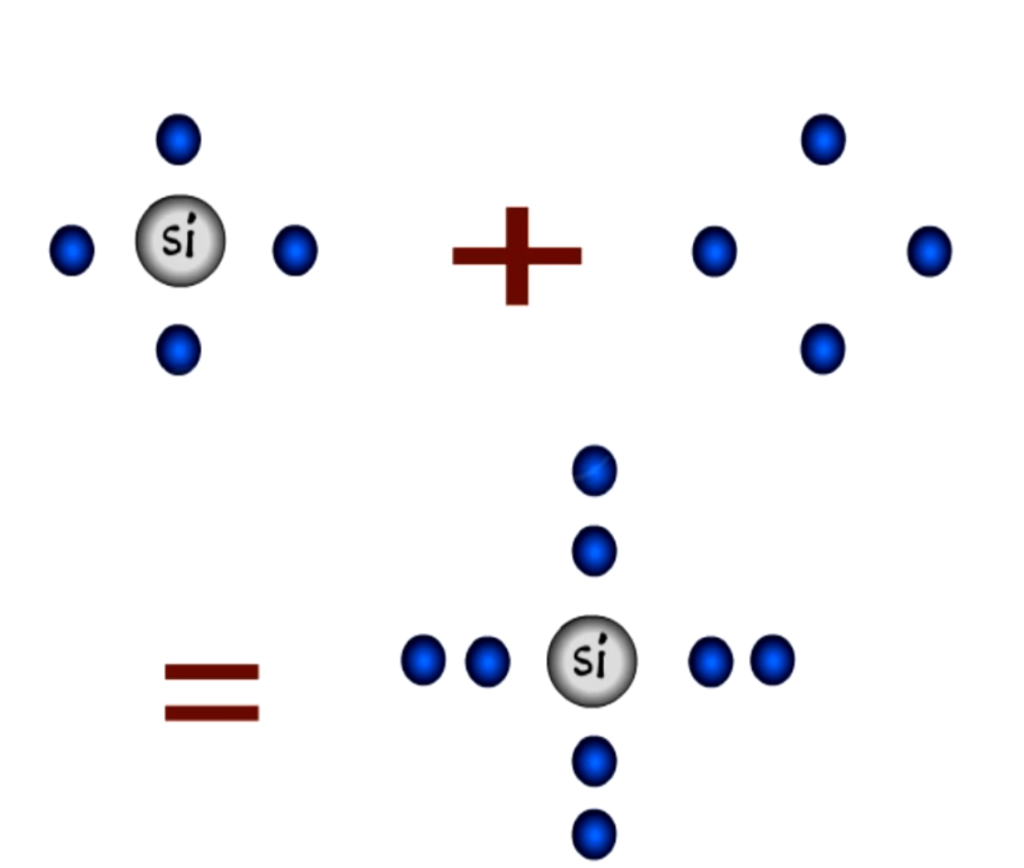
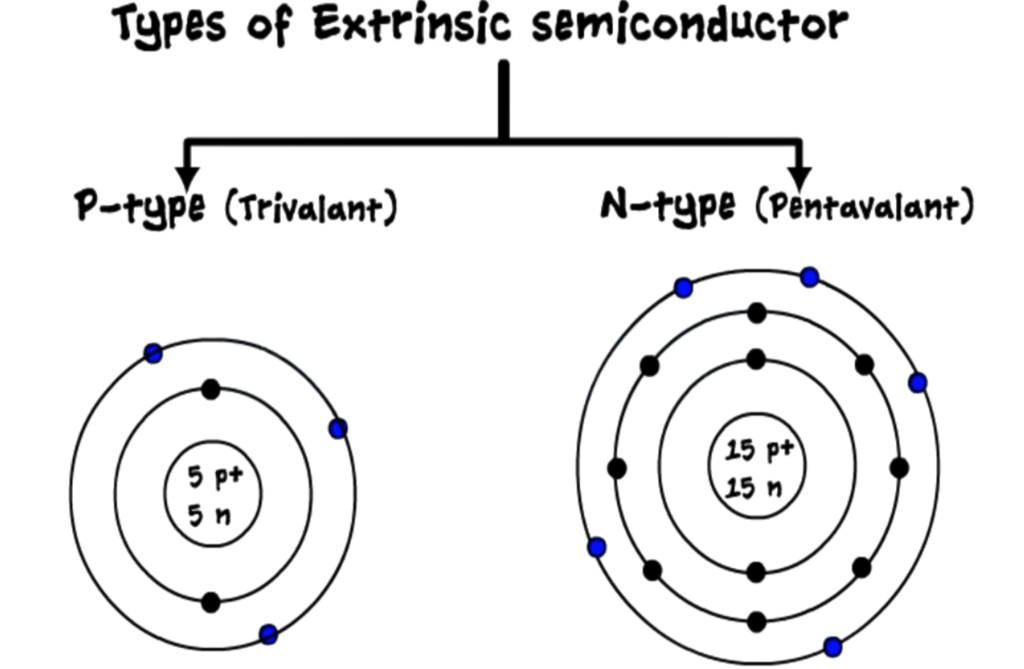
Extrinsic semiconductors
- Created by adding impurities to intrinsic semiconductors in a process called doping.
- Two types of extrinsic semiconductors:
- N-Type: Extra electrons are added, creating negative charge carriers.
- P-Type: Creates “holes” by removing electrons, resulting in positive charge carriers.
Difference Between Intrinsic and Extrinsic Semiconductors
Here’s a quick comparison of intrinsic and extrinsic semiconductors:
| Aspect | Intrinsic Semiconductors | Extrinsic Semiconductors |
|---|---|---|
| Definition | Pure semiconductors without any impurities. | Semiconductors doped with impurities to enhance conductivity. |
| Conductivity | Low conductivity. | Higher conductivity due to added charge carriers. |
| Charge Carriers | Equal number of electrons and holes. | Unequal number of electrons (N-Type) or holes (P-Type). |
| Doping | Not doped; natural state. | Doped with elements like phosphorus (N-Type) or boron (P-Type). |
| Examples | Pure silicon, pure germanium. | Silicon doped with phosphorus (N-Type) or boron (P-Type). |
| Applications | Rarely used directly in electronics. | Widely used in devices like diodes, transistors, and chips. |
How Do Semiconductors Work?
Semiconductors work by allowing controlled flow of electricity. The key is the band gap—a small energy range between the valence and conduction bands where no electrons can exist.
When energy is applied (via heat or electricity), electrons jump the band gap, enabling current flow. This property makes semiconductors ideal for creating transistors, diodes, and integrated circuits.
Quick Table: Types of Semiconductor Materials and Their Uses
| Material | Properties | Applications |
|---|---|---|
| Silicon | Abundant, cost-effective | Chips, transistors |
| Germanium | High-speed conductivity | Optical devices |
| Gallium Arsenide | High efficiency, low heat loss | Solar panels, LEDs |
Applications of Semiconductors
Semiconductors play a crucial role in:
- Consumer Electronics: Smartphones, laptops, and televisions.
- Renewable Energy: Solar panels and wind turbines.
- Automotive Industry: Electric vehicles (EVs) and advanced driver-assistance systems.
- Healthcare: Medical imaging devices and wearables.
- Emerging Technologies: AI chips, IoT devices, and quantum computing.
Semiconductor Materials
The most common semiconductor materials include:
- Silicon: The most widely used due to abundance and cost-effectiveness.
- Germanium: Used in high-speed devices.
- Gallium Arsenide: Ideal for LEDs and solar cells.
Conclusion
Semiconductors are the foundation of modern technology. From powering everyday devices to enabling cutting-edge innovations, their importance cannot be overstated. As technology evolves, so will the role of semiconductors in shaping our future.
FAQs
-
What are semiconductors used for?
Semiconductors are used in electronics, renewable energy, and telecommunications.
-
Why are semiconductors important?
They enable the operation of modern devices and technologies.
-
What materials are used in semiconductors?
Common materials include silicon, germanium, and gallium arsenide.
-
What is the difference between N-type and P-type semiconductors?
N-type semiconductors use electrons as charge carriers, while P-type use holes.
-
What is a semiconductor?
A semiconductor is a material that conducts electricity under specific conditions, making it essential for electronics like smartphones and computers.